The Camire Lab
Description of Research
Our laboratory is interested in the molecular and cellular processes that contribute to and regulate blood coagulation. This dynamic defense mechanism has a major impact on human health and disease as failure of this system to respond appropriately can lead to life-threatening bleeding or thrombosis. We are interested in questions related to the enzymology, biochemistry, and molecular genetics of enzyme complexes involved in blood coagulation. We use a combination of kinetic, biophysical and structural techniques in conjunction with the expression of well characterized recombinant blood clotting proteins to gain insight into how proteins involved in the production of thrombin assemble and function within macromolecular enzyme complexes. Furthermore, we also employ biological models to evaluate hemostasis in vivo and to investigate new therapies for treatment of bleeding disorders.
The generation of thrombin, a key enzyme involved in blood coagulation, at the appropriate time and place is central to this process as inappropriate production can lead to hemorrhage or thrombosis. Prothrombinase is the macromolecular enzyme complex responsible for the production of thrombin. It consists of the serine protease factor Xa, the nonenzymatic cofactor factor Va, anionic membranes and calcium ions. There are several ongoing projects in the laboratory:
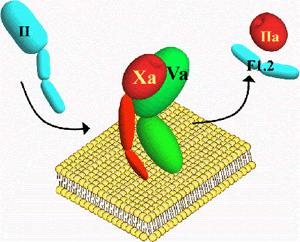
One line of investigation is directed toward understanding how discrete proteolysis of zymogen and procofactor proteins involved in blood coagulation relates to the expression of structural determinants (i.e. exosites, metal binding sites, active site, etc.) important to their function. Specifically, we are interested in studying the conversion of factor V to factor Va and factor X to factor Xa (both proteins are part of the macromolecular enzyme complex, prothrombinase) as model systems to provide insight into the molecular mechanisms underlying the expression of macromolecular binding interactions that accompany transitions from the procofactor and zymogen states.
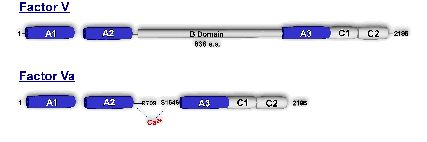
A second line of investigation focuses on defining regions on factor V/Va which directly influence the ability of the cofactor to assemble in prothrombinase. While much is known about factor Va, the precise mechanism by which the cofactor functions in prothrombinase is not clear. Using a combination of strategies, we hope to elucidate which discrete sequences on factor Va contribute to its ability to profoundly influences rates of thrombin generation.
Our group is also interested in serine protease structure/function. Specifically, we are interested in how various structural determinants located on the serine protease domain of factor Xa contribute the specificity of the enzyme and how these regions are influences by various small (i.e. sodium) and macromolecular ligands (i.e. factor Va)
