Lab Resources
Fluorescence Spectroscopy is a major laboratory tool used to resolve the kinetics and thermodynamics of protein-protein, protein-membrane and protein-ligand interactions relevant to the function of the coagulation enzymes. Our strategies rely on the ability to covalently modify one of the interacting species in a specific and discrete way with extrinsic fluorophores that allow for the monitoring of the fate of that species in a complex milieu of reactants. We also employ fluorescent and reversible active site-directed ligands to probe changes or accessibility of the protease active site. Fluorescence resonance energy transfer is an additional approach that we have used to provide an unambiguous signal for the interactions between two differentially labeled protein species.
The instrumentation available in the laboratory for this work is a OLIS rapid scanning fluorescence spectrophotometer with a stopped-flow attachment. This instrument permits single wavelength measurements or the acquisition of entire
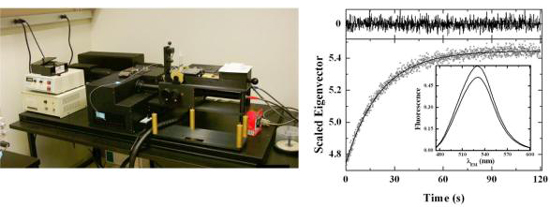
spectra at sub-millisecond intervals in either fluorescence or absorbance mode. Spectrum/time data sets analyzed by Singular Value Decomposition provide a useful method to detect and resolve kinetic intermediates in complex macromolecular interactions.
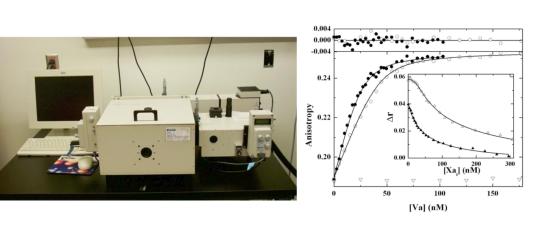
Steady state measurements of fluorescence intensity and/or anisotropy made using a PTI Quantamaster fluorescence spectrophotometer are used for the characterization of binding interactions and for steady state kinetic measurements of fluorescent protein or peptide cleavage
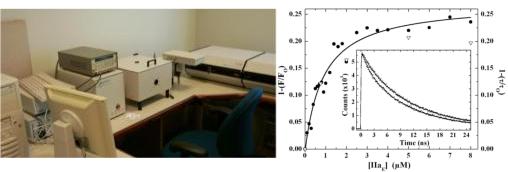
Fluorescence excited state lifetimes are measured with a PTI Timemaster equipped with spectrophotometer with a nitrogen dye laser. This equipment permits measurements in the nanosecond timescale. Lifetime measurements are useful for FRET studies and for studies of protein/fluorophore dynamics.
Thermodynamic constants for the individual binding interactions are determined by Isothermal Titration Calorimetry using a MICROCAL MCS-ITC instrument.
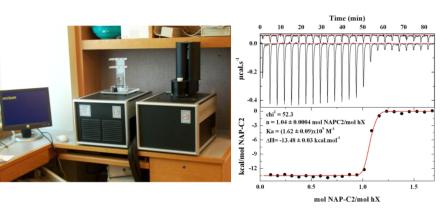
Ligand is titrated into cell containing protein and the binding interaction is monitored by changes in heat flow following every incremental addition of ligand. The resulting isotherm then provides the equilibrium constant and thermodynamic constants for the interaction of interest. Such titrations performed at different temperatures yield the heat capacity for the interaction. An advantage of this approach is that sound thermodynamic information can be obtained without the need to modify the interacting species in any way.
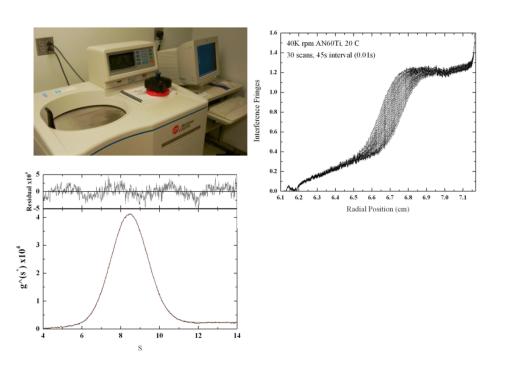
Analytical Ultracentrifugation is performed with a Beckman XLI equipped with absorbance and interference optics. Sedimentation velocity and sedimentation equilibrium studies provide a primary measure of molecular weight and hydrodynamic properties. Both approaches can also be used to directly monitor interactions between species in solution and to establish equilibrium dissociation constants and binding stoichiometries. Differential refractometry can also reliably establish the extinction coefficient of proteins using modest amounts of sample.
Quasi-Elastic Light Scattering (QELS) using a Nicomp instrument is used to characterize synthetic phospholipid vesicles. This method provides hydrodynamic information on particle size based on the angular dependence of scattered laser light. This approach is used to determine the size distribution and homogeniety of the phospholipid vesicle preparations.

Absorbance and fluorescence plate scanning/kinetic plate readers from Molecular Devices are used for routine kinetic measurements.


Because the coagulation enzymes are specific proteases, measurements of enzymic function require assessment of peptide bond cleavage in the substrate protein. While we frequently rely on indirect measurements of product formation, steady state and rapid kinetics are greatly aided by a direct assessment of product formation by SDS-PAGE. This is achieved by Rapid Chemical Quench using a Bio-Logic QFM-5. Reactants are mixed together, aged for the appropriate time, the reaction is quenched and expelled from the instrument into a sampling tube for analysis by SDS-PAGE or other method. This allows us to obtain information of the kinetics of peptide bond cleavage in the protein substrate on the millisecond time scale.
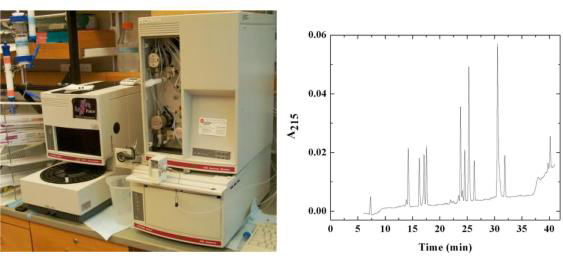
Analytical and preparative reversed phase HPLC with a Beckman Gold Nouveau instrument is used to monitor protein fragmentation, purification of protein fragments for structure/function studies, preparative purification of some recombinant proteins and purification of active site-directed ligands.
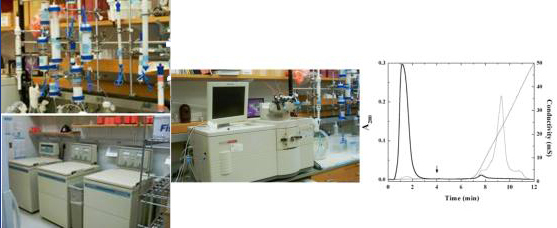
Protein preparative work in the laboratory involves the isolation of a series of coagulation proteins from human blood by conventional biochemical techniques. Activated proteases and cofactors are prepared by proteolytic activation of purified zymogens and procofactors with the appropriate proteases followed by purification of the activated product. Preparative chromatographic separations are developed and optimized using a BioCad Sprint system.

Standard molecular biology techniques are used express recombinant wild type and mutant proteins in sufficient quantities for physical biochemistry work. Smaller proteins, especially inhibitors, are expressed in Pichia Pastoris as a secreted product. High level expression (several hundred milligrams-grams) is achieved by fermentation under controlled conditions in a BioFlo 3000 instrument.
Because of the key role of posttranslational modifications in coagulation protein function, recombinant forms of the clotting factors are expressed in mammalian cells (BHK, HEK 293, AV12) and are purified from conditioned serum-free medium following large scale expression.
